Aberdeen, Scotland and Singapore [January 11, 2021] – TauRx Pharmaceuticals Ltd today announced it has completed patient enrolment of the LUCIDITY study (NCT03446001) ahead of schedule, despite delays due to the COVID-19 pandemic. TauRx is pleased to be presenting its latest trial results at Biotech Showcase™ 2021 during the J.P. Morgan 39th Annual Healthcare Conference 2021. LUCIDITY is the only late-stage study targeting the tau pathology of Alzheimer’s disease. It aims to confirm hydromethylthionine as the first tau-based disease modifying treatment for Mild Cognitive Impairment and Mild-Moderate AD.
Professor Claude Wischik, Executive Chairman and Co-Founder of TauRx, commented “We are delighted by the speed of recruitment which reflects the interest generated by this new treatment approach. We would like to thank study coordinators and investigators, and most importantly the patients who have volunteered to be part of this potentially ground-breaking clinical trial. There is an urgent need for a treatment targeting the tau pathology of Alzheimer’s disease.”
Treatments currently available help with the symptoms for a time, but do not stop the disease from progressing. In earlier Phase 3 trials, hydromethylthionine was found to produce large reductions in progression of both cognitive decline and brain atrophy that depend on the amount of drug that was absorbed (Schelter et al. 2019 DOI 10.3233/JAD-190772).
LUCIDITY was designed to confirm these results in a randomised controlled trial across a broad range of AD disease severity. The trial compares an oral hydromethylthionine dose of 16 mg/day dose against placebo. The primary outcomes are progression of cognitive decline and functional impairment over 12 months measured by standard clinical scales. The trial also aims to confirm that hydromethylthionine reduces progression of brain atrophy measured by MRI scan. Initial results are expected in the first half of 2022.
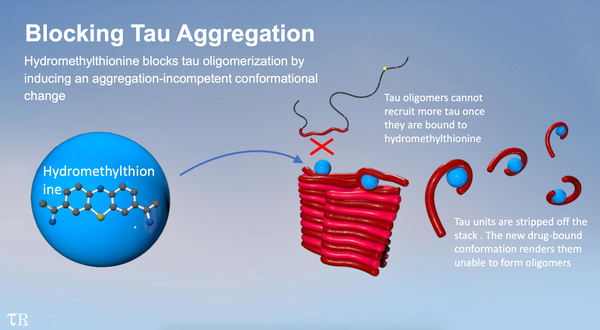
Hydromethylthionine works by blocking the abnormal aggregation of tau protein in the brain that leads to the formation of the neurofibrillary tangles originally discovered by Alzheimer. Tau aggregation is now generally understood to be more highly correlated with clinical decline and reduction in brain metabolism than deposition of amyloid which has been the main target in clinical trials for the last 20 years. If the efficacy of hydromethylthionine is confirmed, it will open up a completely new treatment direction for a devastating disease which has so far defied all efforts to bring it under control.
More information on the LUCIDITY clinical trial can be found at www.LUCIDITYtrial.com and educational materials on the role of tau proteins in Alzheimer’s disease can be found at www.targetingtau.com.
ABOUT TAURX PHARMACEUTICALS LTD
TauRx has focused its research on tau pathology in Alzheimer’s and other neurodegenerative conditions. Hydromethylthionine is currently the only drug targeting the tau aggregation pathology of AD in a Phase 3 clinical trial. It has already been tested in over 2000 patients in Phase 3 trials in both mild to moderate AD, and also in behavioural variant Fronto-Temporal Dementia. Recently published population-based analyses looking at how blood levels relate to treatment response showed similar concentration-dependent pharmacological activity on clinical decline and brain atrophy in both diseases. The predicted optimal dose in AD was found to be 16 mg/day as monotherapy. The LUCIDITY trial aims to confirm the efficacy of hydromethylthionine against placebo in over 500 patients. Results are expected to be available by mid-2022.
The company was established in Singapore in 2002 with the aim of developing new treatments and diagnostics for a range of neurodegenerative diseases characterised by abnormal protein folding and aggregation. The company’s protein aggregation inhibitor, hydromethylthionine, targets aggregates of abnormal fibres of tau, TDP-43 and synuclein proteins that form inside nerve cells in the brain. TauRx’s headquarters are in Singapore and its primary research and operational facilities are based in Aberdeen.
ABOUT BIOTECH SHOWCASE
Biotech Showcase, produced by Demy-Colton and EBD Group, is an investor conference focused on driving advances in therapeutic development by providing a sophisticated networking platform for executives and investors that fosters investment and partnership opportunities. The conference takes place each year during one of the industry's largest gatherings and busiest weeks.
“We are delighted that TauRx will be presenting at Biotech Showcase this year,” said Sara Demy, CEO of Demy-Colton. “Biotech Showcase is a prime occasion for life science entrepreneurs and investors to come together to discover the potential of innovative technologies that will drive the future of drug discovery.”