For Immediate Release. Jan. 11, 2021. Bozeman, MT. Nanovalent Pharmaceuticals has been selected to present at the 2021 Biotech Showcase, a premier investor conference held each year during the week of the JP Morgan Healthcare Investment Week. This year, the conferences will be held digitally, and Nanovalent will have a pre-recorded presentation available beginning January 11, 2021. The Biotech Showcase is designed to get presenting companies and funded technologies in front of potential investors and strategic partners.
Nanovalent CEO, Timothy Enns, said, “It is an honor to be selected by such a strong committee, and to be featured at a major investment forum.” He added, “It is an exciting time for Nanovalent as we advance our science, our company, and our business relationships. I expect to announce significant updates at the Showcase, and in the coming months.”
The process for selection is rigorous and designed to pre-select the companies showing a high likelihood of interest to niche investors. All applications to this prestigious program were reviewed by a panel actively scouting promising oncology-related technologies. For Nanovalent, the acceptance includes participation to present at BIO Investor Forum Digital, January 11-15, 2021.
Please visit http://www.nanovalent.com for more information.
About NanoValent Pharmaceuticals, Inc.
NanoValent Pharmaceuticals, Inc., founded in 2006, is a privately-held company focused on the development and commercialization of a new generation of Targeted Nanospheres (TNS). Working in close collaboration with Children’s Hospital Los Angeles (CHLA), NanoValent aims to develop superior therapeutics for patients restricted by current treatment options and working with other pharmaceutical companies to optimize or expand the utility of additional therapeutics.
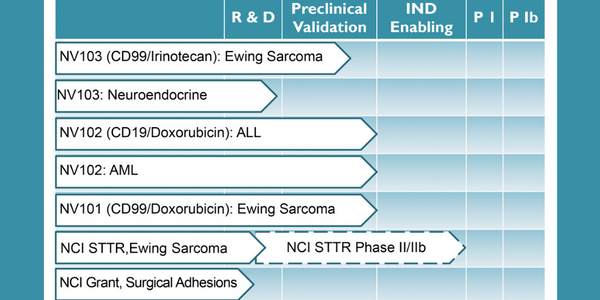
The company’s proprietary platform technology is an important, new, tool that can potentially improve the efficacy and safety of a wide range of therapeutic molecules and biologics. The targeting options and flexibility of the platform make potential applications almost limitless. NanoValent’s leading drug candidate, NV103 (anti-CD99, irinotecan), is approaching phase I clinical trials with initial validating programs including Ewing Sarcoma, Hepatocellular Carcinoma, Neuroendocrine tumors, Acute Myeloid Leukemia (AML), and Acute Lymphoblastic Leukemia (ALL). Other cancer therapeutic candidates include NV101 (anti-CD99, doxorubicin) and NV102 (anti-CD19, doxorubicin).
Funding has come from direct management investment, angel investors, and significant grants from the National Science Foundation, the National Cancer Institute, and the Montana Chamber of Commerce. Please visit http://www.nanovalent.com for more information.