Want to curate the report according to your business needs
Report Description + Table of Content + Company Profiles
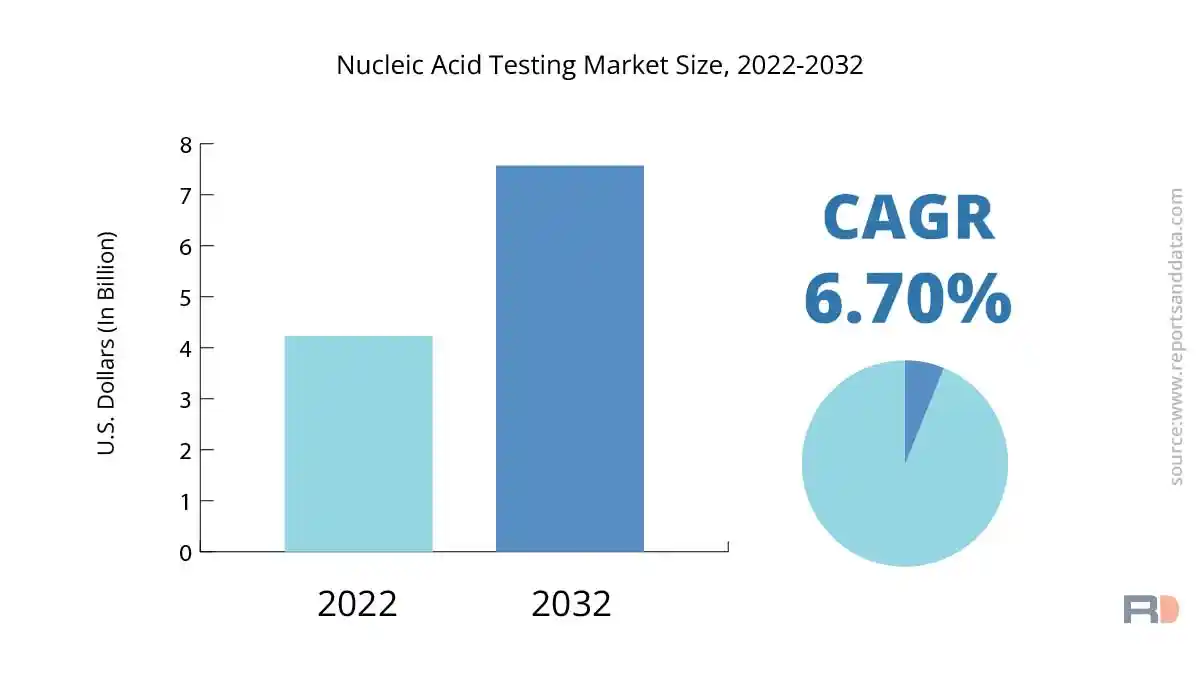
The global nucleic acid testing market size was USD 4.23 Billion in 2022 and is expected to reach USD 7.57 Billion in 2032, and register a revenue CAGR of 6.7% during the forecast period. Rising incidence of infectious diseases and the demand for precise and quick diagnostic tests are major factors driving the market revenue growth. The market revenue growth is being driven by Nucleic Acid Testing (NAT), a technology that is sensitive and specific for identifying viral, bacterial, and genetic diseases in blood samples.
The nucleic acid testing market revenue growth is a result of factors including the rising prevalence of infectious diseases, technological developments in NAT, and rising government initiatives for disease detection and control. To diagnose infectious diseases including Human Immunodeficiency Virus (HIV), hepatitis B and C, and other disorders, the World Health Organization (WHO) has advised using NAT. Moreover, the market revenue growth is driven due to the growing use of NAT in Blood Screening tests. The demand for NAT in blood banks and plasma centers is being driven by the use of NAT to identify viral infections in blood donors.
Moreover, the launch of novel and ground-breaking NAT products, such as portable and point-of-care NAT systems, is expected to drive market revenue growth. These technologies are being used in rural and resource-constrained places because they are affordable and yield results quickly. Moreover, the market revenue growth is being driven by growing implementation of automation in NAT. Compared to conventional NAT techniques, automated NAT systems have several benefits, including higher throughput, less effort, and more effectiveness.
The demand for NAT is also being driven by rising frequency of chronic diseases including cancer and genetic abnormalities. The industry is expanding since NAT is used to diagnose and track genetic diseases and cancer. Moreover, the NAT industry is expanding due to the rising need for personalized treatment. The demand for NAT in precision medicine is being driven by the use of NAT to identify genetic variants in individuals that can impact their response to medications.
The nucleic acid testing market revenue growth is being stimulated by the rising use of molecular diagnostics. The subject of molecular diagnostics, which uses NAT for illness diagnosis and monitoring, is one that is expanding quickly. The nucleic acid testing market revenue growth is being driven by rising usage of molecular diagnostics in oncology, genetic testing, and infectious disease testing. Moreover, revenue growth of the market is being driven by the rising use of point-of-care molecular diagnostics. Point-of-care molecular diagnostics are becoming more popular in clinics, hospitals, and ambulatory care settings because they can produce results quickly and accurately.
However, major factors restraining the market revenue growth during the projected period include high cost of NAT products and absence of reimbursement for NAT testing in some countries. In addition, it is expected that the absence of knowledgeable specialists and the ignorance of NAT in developing countries will impede revenue growth of the market.
In 2021, the PCR-based category held a commanding revenue share in the worldwide nucleic acid testing market. PCR-based, isothermal amplification, microarray, sequencing, and other market segments have been established. Due to its high sensitivity and specificity, which make it a popular choice in the detection of infectious disorders, the PCR-based category represented a sizeable revenue share in 2021. A useful tool in the investigation of gene expression as well as the detection of genetic alterations, the PCR-based approach can detect and amplify even minute amounts of nucleic acid. Also boosting revenue growth in this market niche is the use of PCR-based nucleic acid testing, which is expanding in research and development efforts.
Throughout the projection period, the isothermal amplification segment is anticipated to post the quickest revenue Growth. The rising demand for quick and precise infectious disease diagnostic tests is anticipated to fuel revenue growth. Nucleic acids can be amplified via isothermal amplification at a constant temperature without the use of thermal cycling, resulting in a quicker and more effective testing procedure. The market for this product category is expanding because isothermal amplification is appropriate for usage in environments with limited resources and for point-of-care diagnostics.
Throughout the projected period, the microarray segment is anticipated to experience consistent revenue growth. Genotyping, gene expression profiling, and pathogen detection all benefit from the simultaneous detection of various nucleic acid sequences made possible by microarray technology. The use of microarrays in clinical diagnostics and research and development activities is propelling revenue growth in this market.
Throughout the projection period, the sequencing segment is anticipated to experience significant revenue increase. Market expansion in this sector is being driven by the rising need for high-throughput sequencing technologies for genetic analysis, genomics, and customised medicine. Also, the segment's revenue is growing as a result of the declining cost of sequencing and the accessibility of new sequencing platforms.
Alternative nucleic acid testing techniques including Digital PCR and loop-mediated isothermal amplification are included in the others part (LAMP). Throughout the forecast period, it is anticipated that these methods, which are gaining momentum in clinical diagnostics and research and development, will fuel revenue growth in this market.
Based on application, the market for nucleic acid testing has been divided into segments for infectious illnesses, cancer diagnosis, genetic testing, and others. Infectious disorders accounted for the biggest revenue share of these applications in 2021.
The need for nucleic acid testing in the infectious diseases market is anticipated to be driven by the increasing prevalence of infectious diseases including HIV, hepatitis, and tuberculosis. By assisting in the early detection and diagnosis of infectious disorders, nucleic acid testing enables prompt treatment and disease prevention. Also, the expansion of this market is being aided by diagnostic laboratories and hospitals' growing use of nucleic acid testing.
The sector for cancer diagnosis is anticipated to develop significantly throughout the course of the prediction. The discovery of cancer biomarkers and genetic alterations linked to various types of cancer is frequently accomplished through nucleic acid testing. This market is anticipated to expand as a result of the rising incidence of cancer worldwide and the rising desire for individualised cancer therapy.
Over the projection period, the genetic testing segment is also anticipated to have considerable expansion. Genetic testing, which aids in the identification and treatment of illnesses like Down syndrome, cystic fibrosis, and sickle cell anaemia, frequently uses nucleic acid testing. This market is anticipated to increase as a result of rising need for early and accurate diagnoses of genetic illnesses and rising awareness of genetic testing.
Throughout the anticipated period, the others segment, which includes applications like forensics and Food Safety Testing, is also anticipated to expand. The identification and analysis of DNA evidence in forensics is frequently done via nucleic acid testing. Moreover, the need for nucleic acid testing in the food industry is anticipated to rise due to the increased focus on food safety and quality management.
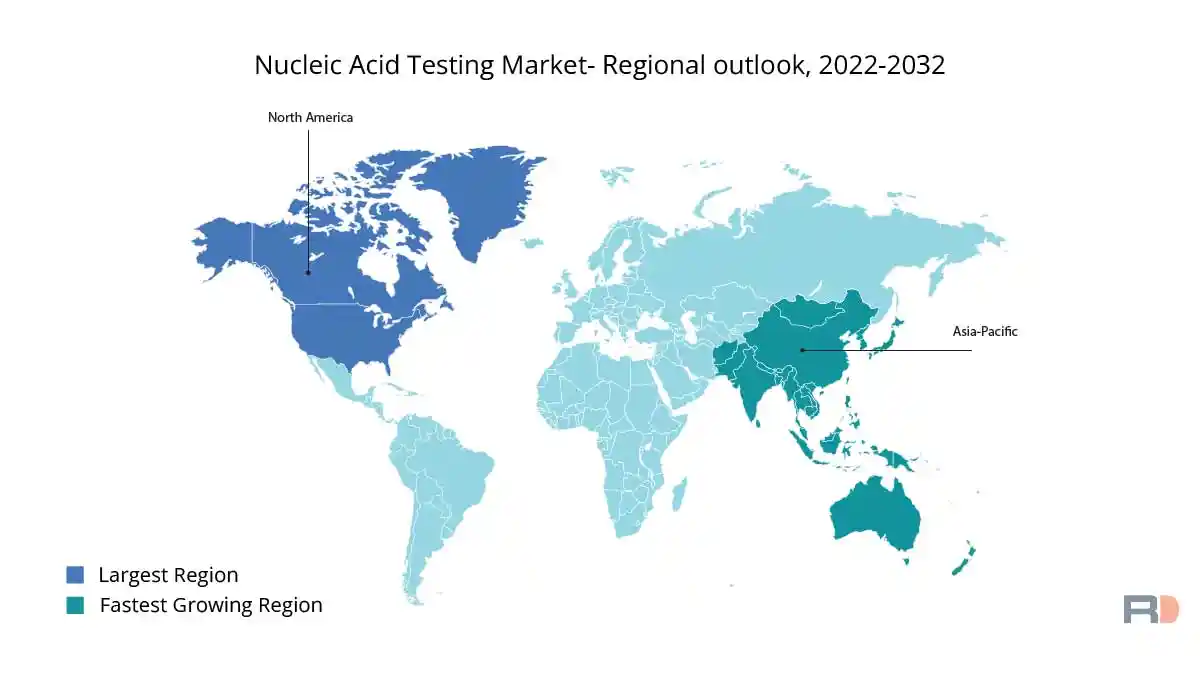
Due to the region's superior healthcare infrastructure and rising investments in R&D, North America held the biggest revenue share in the worldwide nucleic acid testing market in 2021. Nonetheless, the Asia Pacific region is anticipated to have considerable growth over the course of the projection period due to the rising incidence of infectious diseases and cancer, rising healthcare costs, and growing public awareness of genetic testing.
In terms of revenue, the Asia-Pacific market dominated the other regional markets for nucleic acid testing in 2021. Due to factors such an increase in the prevalence of infectious diseases, a rise in the demand for early and accurate disease detection, and a greater understanding of the advantages of nucleic acid testing, the region is predicted to maintain its dominance during the projection period. With their large populations, populous nations like China and India are seeing an upsurge in the incidence of infectious diseases. The governments of these nations are making efforts to upgrade their healthcare systems, which is anticipated to boost demand for nucleic acid testing.
Throughout the projected period, the North American market is anticipated to have the quickest revenue CAGR. The demand for nucleic acid testing in this area is being driven by the increase of chronic diseases including cancer and genetic abnormalities. Also, the market for nucleic acid testing in North America is anticipated to develop due to the rising popularity of personalised treatment, as well as the increased emphasis on early disease detection and disease prevention. Also, the region is seeing an increase in funding for R&D initiatives, which is predicted to spur market expansion.
Over the forecast period, the Europe market is anticipated to expand moderately. The demand for nucleic acid testing is primarily being driven by the rising prevalence of chronic diseases including cancer and cardiovascular disorders in the region, which has a well-established healthcare system. The UK has one of the greatest healthcare industries in Europe because to massive infrastructure spending by the government. Also, the region's nucleic acid testing market is anticipated to grow as a result of the increase in government activities to support illness prevention and early diagnosis.
Some of the major companies operating in the global nucleic acid testing market include:
This report offers historical data and forecasts revenue growth at a global, regional, and country level, and provides analysis of market trends in each of the sub-segments from 2019 to 2032. For the purpose of this report, the global nucleic acid testing market has been segmented based on product type, application, and region.
| PARAMETERS | DETAILS |
| The market size value in 2022 | USD 4.23 Billion |
| CAGR (2022 - 2032) | 6.7% |
| The Revenue forecast in 2032 |
USD 7.57 Billion |
| Base year for estimation | 2022 |
| Historical data | 2020-2021 |
| Forecast period | 2022-2032 |
| Quantitative units |
|
| Report coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments covered | By Product Type Outlook, Application Outlook, Regional Outlook |
| By Product Type Outlook |
|
| By Application Outlook |
|
| Regional scope | North America; Europe; Asia Pacific; Latin America ; Middle East & Africa |
| Country scope | U.S.; Canada; U.K.; Germany; France; BENELUX; China; India; Japan; South Korea; Brazil; Saudi Arabia; UAE; Turkey |
| Key companies profiled | Abbott Laboratories, Bio-Rad Laboratories, Inc, Danaher Corporation, F. Hoffmann-La Roche Ltd, Hologic Inc, Qiagen N.V, Siemens Healthineers AG, Thermo Fisher Scientific Inc, |
| Customization scope | 10 hrs of free customization and expert consultation |
Facing issues finding the exact research to meet your business needs? Let us help you! One of our Research Executives will help you locate the research study that will answer your concerns. Speak to Analyst Request for Customization
Request a FREE Sample here to understand what more we have to offer over competition…
upto20% OFF
upto20% OFF
Want to curate the report according to your business needs
Report Description + Table of Content + Company Profiles
