HyBryte™ Skin Directed Therapy for Cutaneous
T-Cell Lymphoma
T-Cell Lymphoma

HyBryte™ is a skin directed therapy with visible light activation for the treatment of early stage CTCL that appears to be safe and well tolerated in clinical trials.
HyBryte™ may enable patients to undergo more treatments to manage their disease while accumulating significantly fewer risks/toxicities.
HyBryte™ is not yet approved for sale in any jurisdiction.
HyBryte™ Phase 3 FLASH study results published in JAMA Dermatology. Read the publication.
Dr. Ellen Kim, Lead Principal Investigator for the Phase 3 FLASH study, reviews the trial design and positive results.
What is Cutaneous T-Cell Lymphoma?
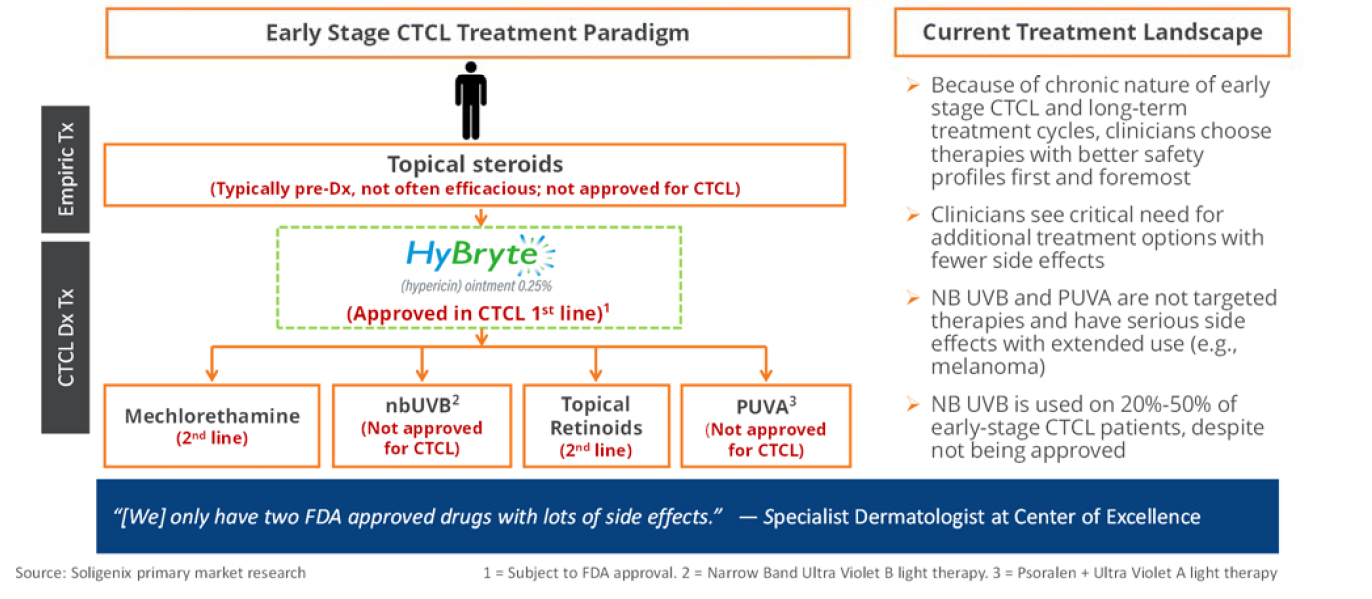
Cutaneous T-Cell lymphoma (CTCL) is a rare type of Non-Hodgkin’s Lymphoma (NHL) which specifically affects T-cells. In its most prevalent form (Mycosis Fungoides), early stage disease is characterized by waxing and waning tumors, lesions and plaques representing migration of the malignant T-cells to the skin. More severe disease (called Sézary Syndrome) is characterized by more systemic disease and has a lower survival rate. Treatment of early stage disease provides symptomatic relief and may slow disease progression. CTCL is treated with off label drugs as well as a number of therapies for second-line treatment and severe disease. CTCL is usually a chronic disease, requiring life-long attention. Therefore, treatment options must be carefully managed to minimize side effects, including potential risks for more serious cancer such as melanoma.
What is HyBryte™?
HyBryte™ (SGX301 or synthetic hypericin) is an ointment containing hypericin, one of the most photosensitive compounds known. HyBryte™ is applied to CTCL lesions in a thin layer, and, after covering the lesion for 18-24 hours, the lesion is exposed to a concentrated visible light source. The hypericin is activated by the visible light and drives the death of the malignant T-cells in the CTCL lesion.
The ointment and light can be applied twice weekly until treatment response or resolution.
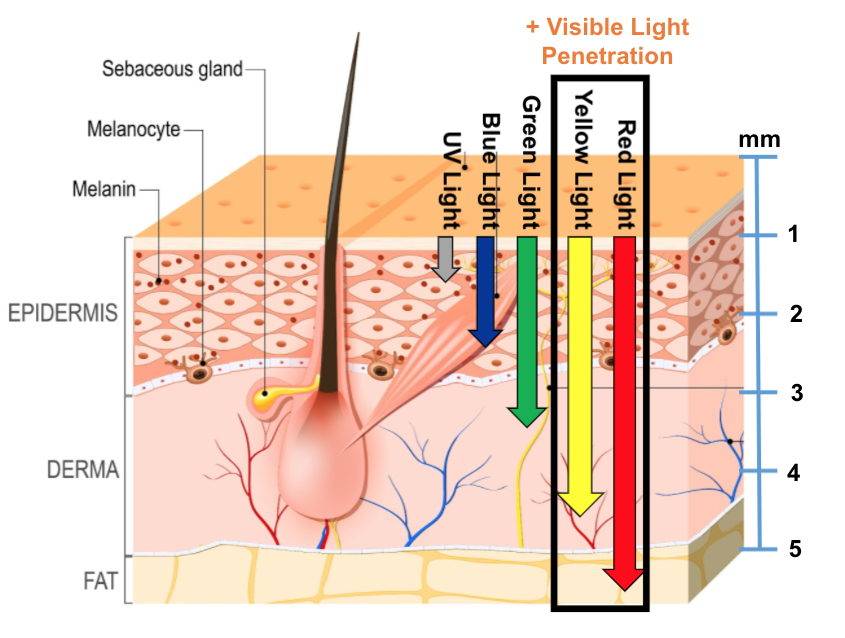
HyBryte™ uses visible light allowing deeper dermal penetration and is NOT associated with a significant risk of carcinogenicity (like other photodynamic therapies which use ultraviolet light).
How well does HyBryte™ treat CTCL?
In a Phase 3 clinical trial, HyBryte™ showed benefit in the treatment of skin lesions of CTCL in as little as 6 weeks. Longer treatment resulted in better responses and longer lasting effects once treatment was completed.
HyBryte™ not only worked against patch lesions of CTCL but also against plaque lesions. Due to the deeper penetration of visible light, it may also be possible to treat variants of CTCL which occur deeper in the skin structure.
What was the design of the Phase 3 trial?
What were the efficacy results of the Phase 3 clinical trial?
In the first double‐blind treatment cycle (Primary Endpoint) ‐ there was a statistically significant improvement in the number of treatment responders (p=0.04) with 16% of the 116 patients receiving HyBryte™ versus 4% of the 50 receiving placebo treatment of their index lesions.
Longer treatments improved response rates substantially.
- Patients receiving 2 cycles of HyBryte™ had a 40% response rate (p<0.0001, n=110)
- Patients receiving 3 cycles of HyBryte™ had a 49% response rate (p<0.0001, n=78)
Patch and plaque lesions responded to HyBryte™ similarly.
- 37% patch lesion response rate after 2 cycles of HyBryte™ (p=0.0009)
- 42% plaque lesion response rate after 2 cycles of HyBryte™ (p<0.0001)
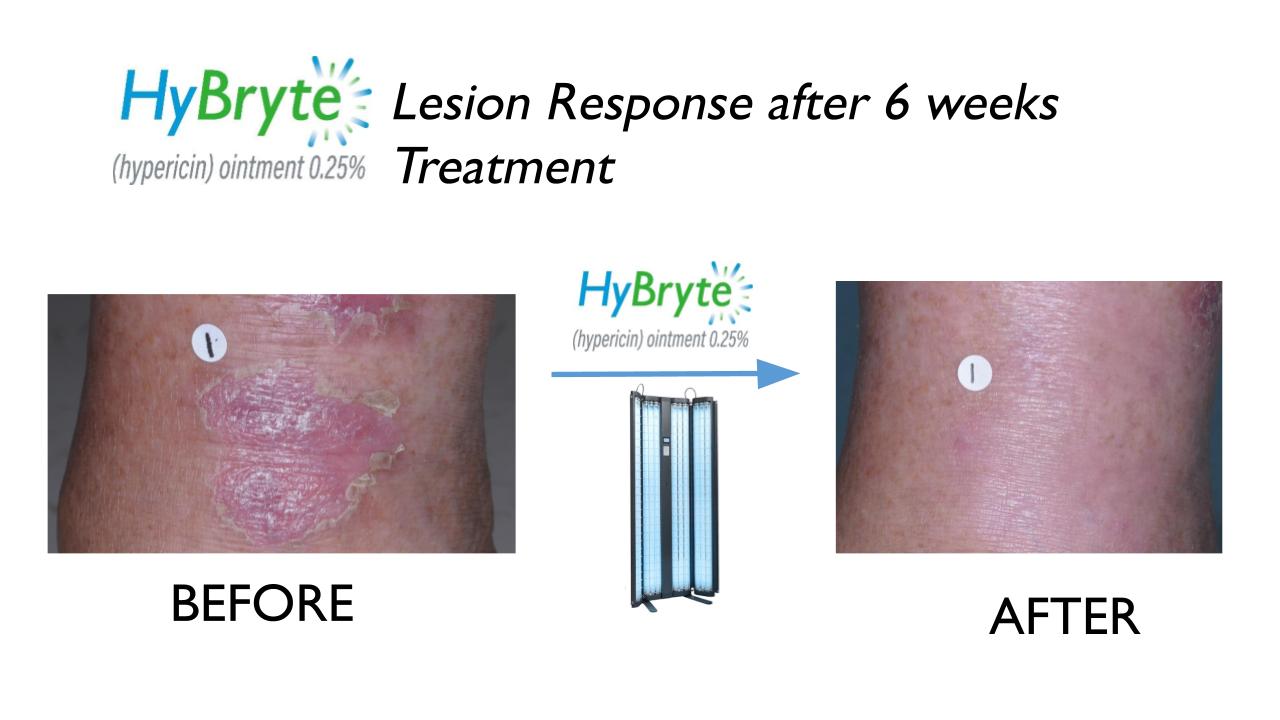
How safe is HyBryte™?
In the largest, multicenter, randomized double-blind placebo-controlled skin directed therapy study in CTCL to date, HyBryte™ was found to have very few serious or severe adverse events. Most reported adverse events were skin related events.
Compared to other, second-line, approved drugs for the treatment of CTCL, this response was shown to be as good or better than other treatments.
What were the safety results of the Phase 3 clinical trial?
- Only 2.4% of AEs were SAEs
- Only 4% of AEs were severe
- Most frequent AEs were skin related events such as pruritus, erythema, hyperpigmentation, burning sensation, dermatitis, which were generally mild or moderate in severity and occurred in 16% of HyBryte™ treated patients vs 10% of the placebo patients
Compared to other, second‐line, approved drugs for the treatment of CTCL, HyBryte™ demonstrated significantly less safety concerns. HyBryte™ treated patients, lower than typically observed in other early stage CTCL trials, with a dropout rate on treatment of only 5%.
Treatment Landscape
What makes HyBryte™ Unique?
- HyBryte™ contains synthetic hypericin as the active ingredient – a compound known for its excellent photosensitivity.
- HyBryte™ utilizes safe visible light – because hypericin is very photosensitive, visible light will activate it. This avoids the need for ultraviolet light, which is also linked to increased cancer risk. Visible light also allows for deeper penetration into the skin.
- HyBryte™ does not cause direct damage to DNA, unlike other photoactive agents.
- HyBryte™ can induce a rapid treatment response, in as little as 6 weeks for some patients.
- HyBryte™ uses visible light – which may facilitate the development of a home-use device for patient convenience.
- HyBryte™ is effective against both patches and plaques, both of which are common in early stage CTCL.
Learn more about HyBryte™
Interested in helping us learn more about HyBryte™? Please sign up here.